na+cl =nacl2
cu20+hno2 = cu2(no2)
fe + 0 = feo2
hci + na = na2h
al + o = alo2
c2 + fe = fec3
hg + o = hgo2
Monday 23 December 2013
Saturday 21 December 2013
Sunday 15 December 2013
nitrate acid and copper
Saturday 14 December 2013
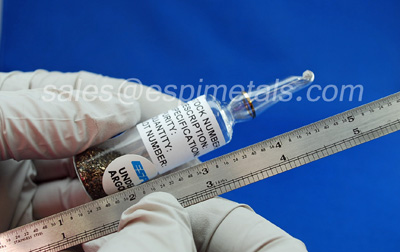
cerium
on earth cerium is almost as abundant as copper (29) and is quite inexpensive especially in the form of cerium oxide widely used as an abrasive powder for polishing glass . cerium metal is pyrophoric witch
meaning that it can catch fire when scratch,file or grind it. in practice that dose not mean the hole lump caches fire but rather that the shavings burn as formed making the material very sparky . not surprisingly this makes cerium useful in liter flints where is highly pyrophoric nature is moderated by alloying it with iron (26).
large lumps of undiluted mischmetal lanthanum-cerium mixtures described under lanthanum (57) are used in movie special effects to create large sparks.cerium has a atomic number of 58 atomic mass of 140 and atomic symbol of ce.
Monday 2 December 2013
the soft metals
the soft metals are known as cu ag au the metals sit in one column on the periodictabal of elements
the real name for them are copper gold and silver these are non magnetic metals
the real name for them are copper gold and silver these are non magnetic metals
Subscribe to:
Posts (Atom)